Electrocatalysts could be an energy game-changer. They could allow us to generate electricity, and produce fuels like petrol and diesel, using naturally abundant substances. Predicting a specific catalyst for any reaction will soon be within reach. It looks like the best days of the alchemist might still be ahead.
By Aditya Sengar, Research Associate in the Department of Bioengineering.
We need more efficient and cleaner energy over the dirty fossil fuels we currently have. The science of catalysis has been key to the energy sector. New catalysts could enable reduced use or abandonment of fossil fuels. It is unfortunate that the biggest bottlenecks for adaptation of such processes are political. Examples are the USA leaving the Paris Agreement on climate change, and China’s great reliance on coal.
An inspiring example
We have seen historically that man-made devastating events can be tackled when global powers work together in a timely manner. I remember as a child reading about the depleting ozone layer. In 1980s, scientists realised that refrigerant chemicals called chlorofluorocarbons (CFCs), used in air-conditioners and refrigerators, caused breakdown of ozone molecules in the atmosphere. This reduces the natural atmospheric shield to harmful ultraviolet (UV) light from the sun. Global agreements like Montreal Protocol in 1987 and Kyoto Protocol in 1997 ensured the phasing out of these chemicals.
Scientists experimented with different catalysts to find ways to produce variations of carbon, hydrogen, and chlorine that are ozone friendly. The plan worked and the usage of new refrigerants (hydrofluorocarbons, HFCs) have allowed the ozone hole to mend. It’s now at its minimum since 1982. Unfortunately, it turns out that these HFCs are potent greenhouse gases and contribute to climate change. Countries are now working to replace HFCs with more environmentally friendly natural refrigerants.
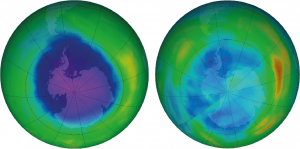
The ozone hole story is also an example of molecular scientists and engineers working closely together to solve a problem. See IMSE’s first briefing paper for more on how this kind of collaboration is essential for progress on today’s grand challenges.
The bigger question
So how could electrocatalysts help governments solve the climate crisis? Wouldn’t it be remarkable to be able to generate power from sunlight using a catalyst? Electrocatalysts offer the potential to do this. They can also produce useful chemicals like petrol, diesel, and ammonia using naturally abundant substances like water, carbon dioxide, and nitrogen, rather than with limited fossil fuels, and without harmful emissions. Let us dig a bit deeper into this.
Electrocatalysts are key to the transition to renewable energy
Electrolysis uses an electric current to force a reaction to occur at an electrode which is also a catalyst. Under normal circumstances, this reaction would be extremely unlikely to occur. For example, using an electric current can be used to split water into hydrogen and oxygen. This happens in an electrocatalytic cell. The hydrogen can be stored for later use in a hydrogen fuel cell. If the original electric current to make the hydrogen derives from burning natural gas (‘blue’ hydrogen) then the hydrogen still has significant CO2 emissions cost. But if the original water molecule was split using renewable electricity (wind, solar) this cost falls to (close to) nil. Currently it’s more expensive to produce green hydrogen than blue hydrogen but this is shifting as energy markets develop.
Another example of a key electrocatalyst is in carbon capture. This is a process in which carbon dioxide from the atmosphere is reduced catalytically to form important products like methane (called green methane), hydrocarbons, carbon monoxide etc. See IMSE’s 3rd briefing paper on the economic and environmental value of this technology for the UK.
Finding the best electrocatalysts
The main research challenges at present are: finding the best catalyst, and scaling up a successful experiments from lab scale to commercial scale.
It’s a great irony that gold and silver, the metals that alchemists wanted to synthesize, have excellent catalytic properties. Finding the perfect catalyst, however, remains a challenge. Major research labs rely on spectroscopy methods to determine catalyst dynamics during an ongoing reaction. These include X-ray photoelectron spectroscopy (XPS) or nuclear magnetic resonance spectroscopy (NMR). The data is used to compare the catalyst dynamics from other samples to find the optimum catalyst.
The advent of supercomputers has been a great help to catalyst science. With more processing power, scientists can model the catalyst dynamics on the molecular level. Instead of relying on experimental data, computational techniques can predict catalyst properties and reaction dynamics from theoretical principles. These techniques include Density Functional Theory (DFT), microkinetic modelling and kinetic Monte Carlo (kMC).
As computational power progresses, the dream of predicting a specific catalyst for any reaction will soon be within reach. How will this speed up the transition to green energy? We’ll find out…
Conclusion
The science of catalysis is almost 200 years old. The 20th century saw an explosion in the industrial use of catalysts, spurred on by rising energy demand. In 2020, the global catalyst market stands at a net worth of USD 35 billion and is expected to reach USD 48 billion by 2027 with a 4.4% annual growth rate. Catalysts enable 90% of US chemical manufacturing processes. They are used to make more than 20% of all industrial products. This means that catalysis has a direct or indirect contribution of 30-40% on the world’s GDP. The catalysis industry produces 20% of the greenhouse gas emissions.
Catalytic processes which enable lower greenhouse gas emissions are starting to penetrate established fossil fuel-powered markets. Processes like Fischer-Tropsch that still take coal as a feedstock to produce synthetic fuel are an active field of research, with hopes to reduce coal dependence by employing biomass as feedstocks. Catalyst industries have also grown hand in hand with the market for production of sustainable energy solutions. Electrocatalysts are key to producing ‘green’ hydrogen and carbon capture. In countries without major renewable energy resources, catalysts might just be the Philosopher’s Stone that saves them from an impending energy crisis whilst addressing climate change concerns.