Gold can make things happen. This has been true since it was first discovered; it looks precious, it’s relatively rare, and it can be easily transformed into items for trade. A symbol of wealth and status for thousands of years, it was originally made into coins in 550BC, and has a history of being used to create valuable items such as jewellery.
Research published by Chemical Engineering PhD student Motaz Khawaji and Professor David Chadwick has demonstrated how gold can make things happen on a chemical level, by using gold particles to create reactions. Their work has recently been featured on the covers of two academic journals, Catalysis Science & Technology and ChemCatChem. Here we take a look at how they use gold nanoparticles and how they could be utilised by industry.
Gold is known to be a relatively stable material, resistance to corrosion and most chemical reactions, and so until recently it was thought to be chemically inactive. However, new research has shown that gold can act as an effective catalyst; that is, it can cause chemical reactions to happen.
Catalysts play a major role in the production of chemicals, polymers and fuels. The vast majority of chemical processes rely on the use of catalysts in the conversion of fossil fuels, biomass and chemical intermediates to more valuable and useful products. Catalysts are also used for environmental applications and pollution control (e.g. degradation of harmful gas emissions from industrial plants and combustion engines)
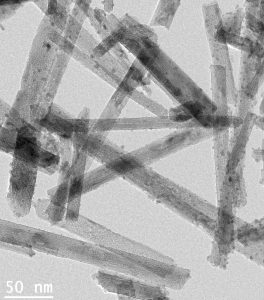
Gold nanoparticles with a diameter smaller than 5 nanometers have the ability to catalyse a number of highly important reactions. One of these is the selective oxidation of benzyl alcohol into benzaldehyde, a clear liquid which is produced naturally by plants. It smells like almond, and is used by the food industry as an additive to cakes and other baked goods
Gold nanoparticles can also be used as a catalyst in making salicylic acid, a chemical routinely used in the cosmetic industry for acne treatments and anti-shine products. Recently hailed a ‘skin saviour’, salicylic can be found in a whole range of products, from serums and mud masks to moisturisers.
PhD student Motaz Khawaji is currently experimenting with gold nanoparticles to better understand how they work, and how industry can effectively use them when chemically engineering products.
“It’s a relatively new area of research so it’s quite exciting. Researchers previously thought gold particles were fairly inactive, which is not the case. My work involves looking at how, why and when gold nanoparticles activate and enable chemical reactions, and where we can apply them.”
By creating novel nanostructured gold-based catalysts and studying their performance in a range of reactions, Motaz Khawaji et al. were able to identify specific structural features responsible for enhancing the catalytic activity. This improvement in our understanding of how the structure and morphology of materials impact the catalytic performance allowed the team to create highly active and efficient gold-based catalysts. The new findings could open the door for the development of new and more efficient gold-catalysed chemical processes, which will eventually lead to better utilization of natural resources, and enhanced atom economy.
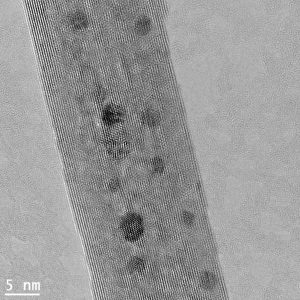
His newly-developed catalyst is being used by a group of PhD, MSc and undergraduate students, who are all running their own experiments testing this catalyst for several applications. One of these applications is the production of clean hydrogen from formic acid, a process which could be used in the future to produce hydrogen as a fuel. Hydrogen is highly flammable and very difficult to store, making it unsuitable as an alternative to petrol, which sits in the tank of a car until it is used. However formic acid is a relatively stable compound, and found in abundance in nature. By fitting cars which gold catalysts, this could enable the chemical reaction of formic acid to hydrogen to take place on-demand inside the vehicle.
In the meantime, Motaz will continue his research to build a case for gold-based catalysts as a stable and cost effective catalyst. He’s been invited to speak at the 8th Tokyo Conference on Advanced Catalytic Science, taking place in August, and is already working on his next research paper.
Motaz Khawaji is a third-year student in the Department of Chemical Engineering at Imperial College London. He previously studied at Michigan State University and the University of Southern California completing a Bachelor’s degree and an MSc in Chemical Engineering. His supervisor is Professor David Chadwick.
Read Au–Pd NPs immobilised on nanostructured ceria and titania: impact of support morphology on the catalytic activity for selective oxidation online in Catalyst Science and Technology.
Read Au‐Pd Bimetallic Nanoparticles Immobilised on Titanate Nanotubes: A Highly Active Catalyst for Selective Oxidation online in ChemCatChem.