In conversation with Lidia Nigrelli, Research Assistant, Osteoarthritis Research Group working within the Centre for Inflammatory Disease

Lidia Nigrelli
What is your research project about and what stage are you at?
Hand osteoarthritis is a condition which can cause painful, swollen and stiff joints in the hands, it can affect the finger joints and thumb joints. Having hand osteoarthritis can make it harder for individuals to carry out everyday tasks. Hand osteoarthritis affects around 2 million people in the UK but there are currently no drugs that slow the progression of the disease or that target pain. Women are at a higher risk for developing hand osteoarthritis than men, and previous work has shown that factors like the menopause may play a role in how hand osteoarthritis develops in women.
Our group, led by Dr Fiona Watt, is called the Osteoarthritis Research Group and we are currently designing a new clinical trial for painful hand osteoarthritis called HOPE-E2 and are in the process of applying for funding. The HOPE-E2 trial stands for Hand Osteoarthritis: a trial of Pain Efficacy of transdermal Estradiol.
A randomised placebo-controlled trial means that participants are randomly put into two groups by a computer – one will receive a placebo (inactive) medication and the other will receive active medication. Neither the study team or the participant will know which type of medication the participant is taking.
The menopause occurs when periods stop due to lowered hormone levels and can cause symptoms such as anxiety, hot flushes, brain fog and disrupted sleep. Hormone replacement therapy (HRT) comes in various forms, including tablets and transdermal forms (patches, creams or gels absorbed through the skin) and can be used to treat menopause symptoms by replacing estrogen and progesterone hormones. Estrogen and progesterone are hormones that are used by the body to regulate processes such as periods and bone strength. Estradiol is a form of the hormone estrogen which is licenced for use in treating symptoms of the menopause. The clinical trial would test whether transdermal estradiol gel can help treat the symptoms of hand osteoarthritis and how this might work.
What did you want patients/the public to help you with?
We wanted to hear from those affected by osteoarthritis, including hand osteoarthritis, to help us shape and improve our research proposal. This is because we want to ensure that our communications with potential trial participants includes the right information, and that all eligible individuals have an opportunity to take part in the trial.
Who did you involve and how did you find them?
In July 2024, we held a public advisory group (PAG) meeting with public contributors, in collaboration with the Imperial Clinical Trials Unit. 9 public contributors took part in the HOPE-E2 PAG session and were recruited from a number of groups across England such as the OsteoArthritis Research Voluntary Interested List (OARVIL) involvement registry.
To ensure that we involved people from diverse backgrounds, a questionnaire which collected information on demographic characteristics including age, sex, race, disability, sexuality, educational level, location and lived experience of osteoarthritis was included in the invitation to potential contributors (respondents could opt in or out of providing this information). Based on these characteristics, respondents were selected and invited to participate in the session, ensuring that the group would be as representative as possible of the research population (people with hand osteoarthritis living the United Kingdom). Of the 9 public contributors involved in the session, most but not all, had lived experience of hand osteoarthritis or other forms of osteoarthritis.
What kind of activity/activities did you undertake to gain insights into your research from public contributors?
We held an online discussion session about the proposed design of the trial. The session started with an introduction to the research area and reason for the trial. We then explained what the HOPE-E2 trial would be like for the participants. Approximately 230 post-menopausal women with painful hand osteoarthritis would be recruited to the trial and would test the HRT medication for 6 months. Participants would be randomly put into a placebo group or HRT group, with both groups continuing with their usual hand osteoarthritis treatments, for example painkillers and exercises. The placebo group would receive a placebo (inactive) medication and the HRT group would be given the HRT medication. The trial will last 8 months in total. Participants would rate their hand pain daily for 14 days before each study visit, complete questionnaires about their hand pain and function, and have blood tests. At the end of the trial, medications would be gradually reduced before stopping.
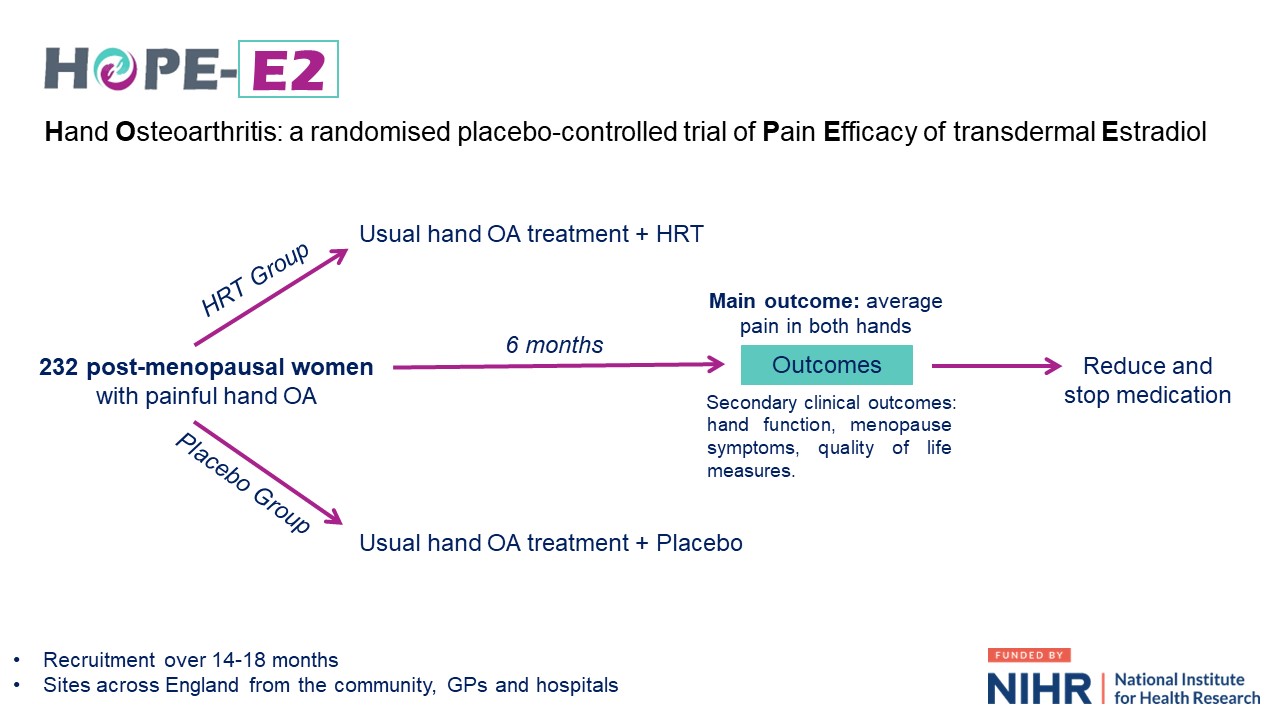
An overview of HOPE-E2 from recruitment to the end of the clinical trial, as described to the public contributors during the PAG session.
All participants would have blood tests to measure inflammatory markers and hormone markers, and as a safety measure to check for any side effects of the HRT treatment. Participants will have up to 7 study visits over 8 months, including a screening visit and final safety check at the end of the study. Some visits can take place by phone instead of in person.
Was any training/briefing provided to the people you involved?
Before the online session, all contributors received a summary of the proposed research. The agenda included a broad, facilitated discussion on key areas, with time at the start and end for initial comments, thoughts and any other ideas.
As this session took place in collaboration with Imperial Clinical Trials Unit, we were fortunate to have the support of Marie Miller who acted as the session’s facilitator and is the Public Involvement and Engagement Lead for the Unit. The Imperial Clinical Trials Unit is involved with the designing and running of clinical trials at all stages at Imperial College London and Marie is experienced in conducting public involvement events so provided helpful guidance for the organising and running of the session.
How did your research change as result of the public involvement insights?
The key highlights raised by the public contributors are listed in the following sections:
Aspects of the trial design that were strongly reinforced:
- Changing the gradual reduction in dose interval for the estradiol gel from 3 to 6 weeks.
- Including worst hand symptoms and duration of these symptoms in our analysis.
- Informing participants what treatment they received at the end of the trial.
- Asking participants to submit daily pain scores by phone link, which they felt was easy to do (though they also requested paper alternatives).
- Covering travel expenses for in-person visits, including taxis.
- Conducting optional sub-studies, separate to the main trial but still carried out by the study team, to look at sleep and markers in blood. The contributors were comfortable with the concept of these sub-studies but emphasised that they would not want these blood tests to be carried out at their GP practice.
Areas of the trial design which needed further consideration or development:
- Some cultures may view HRT as unsafe or unnecessary, and cultural or educational barriers could be greater barriers than language to participation.
- Identify role models and community groups to improve recruitment from underserved communities.
- Not everyone will be able to read translated sheets, sometimes videos and group meetings might be more useful.
- Organise further public contributor advisory sessions like this throughout the trial.
We are now proposing to work with 2 grassroots organisations who are focused on working with community groups, and a community engagement agency that develops communication strategies and materials suitable for different audiences. In our funding application we have ensured that there would be funds available for working with these organisations throughout the trial.
Details of the trial design that we will modify and/or highlight to our participants:
- Make participants aware that they can bring companions to study visits.
- Reduce the questionnaire time from 30-40 minutes to 20-30 minutes at the baseline, 12 week and 24 week study visit sessions, and test the questionnaires with diverse individuals to better understand completion times.
- Ensure there are both digital and paper options for completing study questionnaires.
- Be clear about where to apply the HRT gel and why a gel would be used instead of a HRT patch or tablet, including clarifying that a gel format makes it easier to modify the dose at the end of the trial.
- Give clear guidance about whether other treatments or exercise could be used safely during the trial, and how these will be controlled and monitored. An information pack at the end of trial was also supported.
- Explain to participants how they could access the medication when the trial was over, if they wished to (and how results from the trial would reach the NHS).
- Gathering sleep data using wearables – a comfortable bracelet style option with minimal ‘tech’ input from the user was preferred over a ring style wearable device.
- Focus on designing any sub-studies to not include imaging (our plan for advanced imaging was not supported due to concerns about safety, comfort, time and cost).
The study team also noted that the individuals who choose not to participate in the clinical trial will have very different views on the safety and acceptability of HRT medication, compared to those who decide to take part. Though not the main purpose of our trial, it was recommended we should try to find ways of understanding why.
After the session, a detailed record of all the comments, responses and discussion from the session was made, and the HOPE-E2 research proposal was carefully refined to reflect and action many of the points raised during the PAG session.
What impact did the public involvement have on the people involved?
At the end of the session, contributors were asked if they supported the idea of the participating in the study and the majority of the participants said yes. We also received positive feedback about the session. One participant stated that the session was the best organised online meeting that they had attended, and another said that it was very reassuring that people with lived experiences were being consulted on the trial design.
Several contributors also expressed their willingness to remain involved with future advisory group sessions relating to this research. We intend to work with them again, and also others so that we can continue to ensure that our planned research aligns with the needs of people living with hand osteoarthritis. It was encouraging to hear that individuals feel reassured that sessions like this one take place to shape a trial’s design – it highlights how important public involvement in research is.
What was the most challenging part of doing public involvement in research and how did you overcome it (or not)?
Ahead of the session, it felt hard to balance respecting the privacy of potential contributors versus asking a lot of questions about their personal information/demographic details (to ensure that we had a diverse and representative group of contributors in the session). It was important to explain why we were asking for that information so they could feel comfortable with how it was being used. We found that being clear and transparent from the start of their involvement built trust between the public contributors and the research team.
We also needed to establish whether it was best to hold the session in person or online, as both options had their pros and cons. In the end, the decision to hold the session online seemed to work well for everyone, as some contributors preferred to communicate their ideas via the chat, and this helped us to gather as many comments as possible.
What advice would you give to other researchers embarking on public involvement in research?
Our best advice would be to give yourself lots of time to organise and run your public involvement event. Recruiting and selecting individuals to be contributors can take some time and it is also important to make sure that the format of the session is suitable for the contributors and the discussion topic.
Consider what information your contributors would need to understand about the research area and be able to fully contribute to the conversation. We navigated this by providing a summary of the proposed research in advance and on the day, gave a presentation to introduce the research area and the trial. During the session, we followed an agenda to ensure that all areas were covered, whilst also keeping to time.
We were grateful to have the support of the Imperial Clinical Trials Unit team for our public involvement session. Seeking guidance from colleagues with previous public involvement experience is recommended for anyone embarking on public involvement in research.
So, what’s next?
At the moment, we are continuing to apply for funding to support this clinical trial. We are committed to incorporating public involvement in our research and as the trial develops, we will continue to involve people with lived experiences to ensure that our efforts include the voice of people living with hand osteoarthritis.
In our research proposal we are working with a public co-applicant to support our study development and another co-applicant of the research proposal will lead all aspects of the Patient and Public Involvement for the trial. We have also calculated and proposed funds for a trial oversight group consisting of 3 public contributors (our public co-applicant and 2 other independent members) who will meet multiple times throughout the trial. Additionally, we have requested funding for 3 further public advisory group sessions at the start, middle and end of the trial to advise on all aspects of the trial’s delivery and communication of the findings from the trial.
Finally, we want to say a big thank you to all of the public contributors who took part in the session and to everyone who helped us to run the session!
The study team members present during the session were: Fiona Watt (presenter and researcher), Marie Miller (facilitator and Public Involvement and Engagement Lead), Lidia Nigrelli (researcher and record keeping for the session) and Verity Leeson (point of contact for participants and Imperial Clinical Trials Unit representative).
