- My Imperial Global Development Fellows Fund Placement at Imperial
- Researchers and community members working together to shape research on respiratory infections in young children
- HOPE for Hand Osteoarthritis
- Having an Impact with Public Involvement in Paediatric Intensive Care Research
- Public engagement and involvement at the Cardiomyopathy UK conference: When researchers and the public meet
- Achieving more through public involvement in antimicrobial stewardship
- Involving women in the design of maternal cardiovascular research
- Public involvement in prematurity research at Imperial Women’s Health Research Centre
- The Young People in Psych Research Group: helping scientists iMAGine better research for self-harm
- What’s New and Promising in Neuropathic Pain Research? Involving Patients in Research Co-Design
In conversation with: Donna Kennedy, Clinical Specialist Hand Therapy (Imperial College Healthcare NHS Trust) and Postdoctoral Research Fellow (NIHR Imperial Biomedical Research Centre). Working in collaboration with Prof. Andrew Rice, Dr Harriet Kemp and Dr Whitney Scott within the Pain Research Group led by Prof. Andrew Rice.
What did you do?
The Pain Research Group investigates neuropathic pain in the context of infectious diseases, diabetes and nerve trauma. We undertake patient profiling studies, which include cognitive, psychological and physical measures such as skin biopsies and quantitative sensory testing. Previously, our group’s public involvement work has been very study-specific and has not typically engaged patients early in the research cycle. In addition, while we recently produced a group video and have begun to build our group website, we haven’t had the benefit of involving patients in the development of these communication tools to ensure they are fit for purpose.
Therefore, to improve patient engagement in, and co-design of, our group’s research activity, we hosted two patient workshops. Workshops were held in our lab at Chelsea & Westminster Hospital, and were attended by 18 patients who were of different ages, genders, ethnicities and were living with neuropathic pain caused by a variety of medical conditions. To accommodate peoples’ different schedules, we held one workshop in the early evening and the second in the early afternoon.
Two patients, both of whom had been involved in previous research projects, were heavily involved in the planning and designing of these workshops. They were involved right from the beginning, when we first put together our Public Involvement Grant funding proposal for this idea. These patient co-applicants provided advice regarding the content and style of our event promotional material and advised on the timing of activities and physical movement of participants through the lab space. At each session, one of the two also shared their experience of participating in the co-design of research, helped to facilitate our group discussion and answered any questions from the attendees.
Both workshops involved a tour of our labs, demonstrations of our pain and sensory testing equipment, viewing our group’s neuropathic pain research video and showcasing our website. A presentation on the latest research developments in neuropathic pain provided excellent background for a frank and inspiring group discussion where patients shared their concerns, priorities and suggestions for our work and engagement activities. Midway through the session, we enjoyed light catering. This break allowed time for the participants to get to know one another and enabled more personal interaction between attendees and the Pain Research Group team.
What were you trying to achieve?
We had several aims for our engagement activity.
Firstly, we wanted to identify patients who would be interested in participating in the group’s future research activities as patient co-designers. We envisioned this would include helping us to identify relevant and important research questions, as well as informing the way we design, conduct and share our research. We were keen that these initial workshops weren’t a “one-off” and aimed to develop a patient partner panel to ensure longevity in our relationships with patients and the public.
We were also keen to improve our communication strategy. This included reaching and engaging patient groups that are less often heard and getting feedback on the best methods for communicating with patients about our studies and the measures that we use.
Lastly, we wanted to ensure our group’s digital content, including our group’s website, video and social media channels, were accessible, relevant and useful to our patient and public audiences.
Who did you involve and how did you find the right people?
We used a few approaches to engage people. We initially thought we would advertise using a poster but then dismissed this idea as we were concerned that we might not reach the patients we were targeting; those with neuropathic pain.
The Pain Research Group wrote to patients who had previously participated in their research studies and had consented to be contacted in the future. In addition, invitation letters were given to patients by the Pain Clinics at Chelsea & Westminster. This ensured patients with a variety of neuropathic pain conditions and patients who hadn’t previously been involved in research were invited to take part in our workshops.
We wanted to engage patients from diverse backgrounds and with diverse medical conditions, knowing this would add to the richness of our sessions and improve the representativeness of the feedback we received. It was very helpful having our patient co-applicants involved in the project as they were able to advise on the best ways to share the invites and encourage people to join.
In total, 18 people responded to our invitations. We then did all we could to facilitate their involvement, for example offering a choice of meeting times, catering for dietary restrictions and reimbursing for travel expenses, including taxis. We were delighted that all 18 respondents participated on the day!
We wanted to ensure that the patients attending would feel comfortable taking part in group discussions during the workshops, while being confident that their privacy was being respected. We asked attendees to wear name tags (first name only) to facilitate communication and asked those who were happy to be included in photographs to sign consent forms. At the start of the session, we provided information packets that shared the aims of the session and contact details for the Pain Research Group participants. We identified a facilitator for each session who posed questions to the group and attempted to ensure all participants had the opportunity to be heard. Those researchers not facilitating the session kept notes to ensure we captured the salient points from each session.
Were the people you involved given any training?
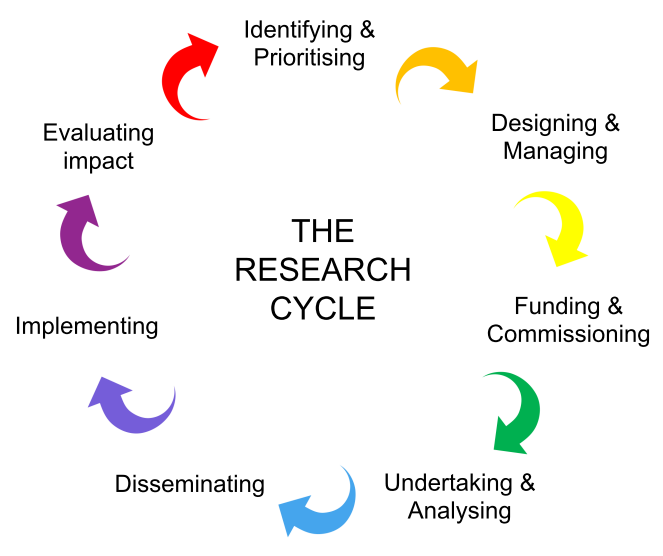
The Public Involvement Resource Hub on the PERC website was very useful for developing tools and handouts for the workshops. We started our workshops with a slide of the research cycle and used this to discuss how patients and the public might be involved in our group’s work across the research cycle.
During the lab tours, we demonstrated our testing equipment and talked though our most common procedures.
Did you achieve what you set out to do?
Yes! Attendees openly shared their concerns and priorities for our group’s research. They emphasised the need for research findings to be shared with patients, particularly with regards to whether/how the findings will change anything in ‘the real world’. They also identified methods to improve our group’s video and website to make them more engaging, useful and relevant to our patients. Alternate methods and routes for engaging patients in our research studies were also put forward, such as with video messages at GP surgeries and through patient support groups.
What’s more, all 18 of the workshop attendees have confirmed they would like to continue to be involved as patient partners in future research projects!
Participant discussion was rich and informative:
- ‘You need a patient in the video sharing their story—what pain feels like and how it interferes with life from their point of view’
- ‘The video helped make the research team seem approachable’
- ‘A key priority for pain management is to help people to re-engage with life’
- ‘Patients want to feel empowered and that they have a sense of control over their life.’
- The group expressed that improving access to helpful treatments is a priority; the current system ‘doesn’t work’.
- It was also highlighted that people often feel like their pain is dismissed by GPs and that they are made to feel like they’re “crazy” for having chronic pain; healthcare providers need to better communicate pain isn’t ‘all in your head’
- And research methods that can capture people’s stories of living with pain alongside their pain ratings was said to be helpful.
We are now in the process of adopting this feedback, improving our video and further developing our group’s website. This work has also supported three funding applications, two of which has been successful.
What impact did this activity have on the people involved?
During and following the pain workshops, we received very positive feedback from the people who attended. We were delighted by their enthusiasm for partnering with us in future co-design activities, which suggests that they truly enjoyed the experience and could see value in their role as a research co-designer.
As a researcher, delivering the pain workshops was an important learning experience. Researchers can easily become accustomed to the vast amount of time it takes to design and deliver a research study. However, particularly in pain research, there are people waiting for research findings that will help their debilitating condition and improve their quality of life. The workshops were a stark reminder that we mustn’t be accepting nor complacent regarding the slow translation of research findings into patient benefit but must always strive to minimise research waste and accelerate knowledge translation.
What was the most challenging part of doing Public Involvement and how did you overcome it?
As clinical academics, the primary challenge for activity of any kind, beyond patient care and research commitments, is finding time. It was challenging for us to find dates and times that worked for the team to deliver the events. In addition, with no administrative or event delivery support, planning, preparation and delivery of the event was hugely time consuming.
The workshops were a success because of the teamwork involved in planning and delivery. We hosted a diverse group of participants, engaged in stimulating and informative discussions and developed relationships with patients who are now keen to partner with us in the design and delivery of future projects. This wouldn’t have happened without a committed team.
What advice would you give others interested in doing something like this?
I would highly recommend it. It was a wonderful opportunity for our research group and our patients.
Be clear about what you want to accomplish from the events. One of the nice things about running two workshops is that we could learn from our first event and tweak our plans for the second event. Like writing a thesis, planning a good workshop takes three times longer than anticipated!
So, what’s next?
We intend to co-write an article or blog with one of our patient co-applicants, sharing highlights of the workshops and promoting further patient engagement with our group. In addition, our patient partner panel is continuing to grow with enthusiastic patient co-designers!

Thank you for so clearly sharing your experience on “What’s New and Promising in Neuropathic Pain Research? Involving Patients in Research Co-Design”
I have begun to experience neuropathic pain in my feet and toes due to diabetes 2. Should you require more PPI in this study I would like to be involved; alternatively please keep me on your mailing list so I an follow any developments. I am concerned about the condition.
Kind regards
Hazel
Thank you for your comment Hazel. I will ensure this is passed onto the researchers and, if you would like us to get in touch with you about other opportunities for public engagement or involvement relevant to diabetes-related pain, please send me an email on p.pristera@imperial.ac.uk and I will add you to our distribution list. Best wishes