 Dr Anna Klöckner is a Marie Skłodowska-Curie Fellow in the Department of Materials. Her research focuses on finding novel treatment strategies to overcome the problem of antibacterial resistance. She works within the Stevens Group and the Edwards Group (Microbiology, CMBI).
Dr Anna Klöckner is a Marie Skłodowska-Curie Fellow in the Department of Materials. Her research focuses on finding novel treatment strategies to overcome the problem of antibacterial resistance. She works within the Stevens Group and the Edwards Group (Microbiology, CMBI).
In our latest blog post, Dr Klöckner shares more about her research and the fight against a potential global health crisis.
Can you tell us more about your research area?
The discovery of antibiotics was one of the milestones in medicine. By preventing and treating bacterial infections, these drugs saved millions of lives. In the Golden age of antibiotic discovery (around 1940-1960), many antibiotics were discovered and introduced in the clinic with the misleading assumption ‘to close the book of infectious diseases’. Instead, bacteria have been striking back by getting more resistant to antibiotics and making these once-powerful weapons blunt. Infections caused by multi-drug resistant bacteria often require higher antibiotic concentration and more prolonged treatment courses that come alongside severe side effects for the patients.
With the challenges of finding new antibiotics alongside fewer investments in R&D, we may head towards the next global health crisis caused by multi-drug resistant bacteria. It is now vital to use multidisciplinary efforts to find new ways to treat bacterial infections. Over the last few years, more and more researchers have started investigating bioengineered drug delivery systems in which drugs are encapsulated into particles or scaffolds as an alternative to conventional antibiotic therapy.
What are the main aims of your current research?
Finding novel treatment strategies to overcome the antibacterial resistance problem is a significant team effort. As a microbiologist, I experience difficulties finding new antibiotics, and I know the importance of looking at the problem from different angles.
Over the past years, I have been working on bioengineered drug delivery systems to make our existing antibiotics more efficient. The treatment of multi-drug resistant bacteria often requires a more prolonged and harsher antibiotic course, which can cause side effects and interfere with the patient’s microbiota.
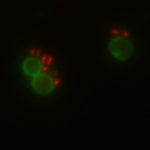
In my research, I focus on drug delivery systems made of multiple compartments that can be loaded with an antibiotic. They look like a flower and act like little trucks carrying goods. Thereby, the trucks protect the cargo from the environment and vice versa. A significant drawback of antibiotics is that they do not selectively kill harmful bacteria and often affect our good microbiota. To avoid that, it is vital to release the antibiotic from the drug delivery system only at the side of infection.
The beauty of our drug delivery system is that an on-demand release mechanism was introduced, which is triggered by molecules that are only produced by pathogenic bacteria. This guarantees a very local antibiotic exposure which protects most good bacteria in the human body. Over the last years, more research has shown that using a combination of antibiotics to treat pathogens often leads to a better outcome than single antibiotic therapy.
The difficulties in antibiotic combination therapy are that both drugs simultaneously reach the side of infection in a high enough concentration. Due to the multiple compartments of our system, it is straightforward to deliver numerous medications in the same area, guaranteeing the bacteria’s exposure to both drugs at the same time. Therefore, drug delivery systems can provide a promising approach to carrying multiple antibiotics and releasing them only where needed. They would be safer and more efficient than their free antibiotic counterparts.
How could this research potentially benefit society?
The Covid-19 pandemic showed once more how devastating a global pandemic can be for society and economics. Before discovering antibiotics, bacterial outbreaks were frequent. With the plaque holding the dark record as one of the most fatal pandemics in human history, we should not underestimate the threat caused by bacteria. With antimicrobial-resistant bacteria on the rise and the challenge in discovering new antibiotics, there is a chance that history will repeat.
The last decades revealed that the golden age of antibiotic discovery is long over and not many new antibiotics made their way into the clinic. Bioengineered antibiotic delivery could help overcome the limitation of our currently existing antibiotics and use them more efficiently. The drug delivery system I am working on is versatile and tailored towards a specific bacterial infection. The delivery of multiple antibiotics and the local release of drugs antimicrobial-resistant bacteria could be targeted easier by simultaneously preventing the interruption of the patient’s healthy microbiota. Therefore, drug delivery systems are a promising way to improve our current antibiotic treatment regime and help to solve the antimicrobial-resistant crisis.
What are the next steps in your research? Are there any challenges ahead?
We are in the early stages of developing this type of drug delivery system, where we showed the triggered release of multiple antibiotics and the resulting antibacterial activity in a laboratory environment. We are currently tailoring the drug delivery systems towards specific bacteria classified by the World Health Organisation as critical due to their resistance to antibiotic therapies. Therefore, new release mechanisms, drug combinations and materials for the assembly of the compartments are under investigation.
The immediate next steps would be to test the drug delivery system in an animal infection model to see whether the antibiotic delivery works in a more complex environment. The longer-term challenges are upscaling the production without losing the quality of the drug delivery system by keeping the production costs low and finding the ideal route of administration.
Overall, there might be a bumpy and long road ahead of us before this type of drug delivery system makes it into a real-world application. Still, if that means we are one step closer to overcoming the antimicrobial-resistant problem, it is worth it.