
Name: Ramin Jannat
Position: PhD student in the Department of Materials.
Research Group: Professor Mary Ryan
Research Focus: Understanding lithium-ion battery degradation – key to improving technologies like laptops to e-bikes.
What inspired you to study for a PhD?
I always knew that I wanted to do a PhD because I loved the idea of having a specific research question and dedicating time to answering it (or trying to, at least!). I did my undergraduate degree in Chemical Engineering at UCL, and my master’s project, supervised by Dr Yang Lan, revolved around investigating the colloidal stability of coronavirus-like particles, a highly relevant project during the pandemic.
The only question I had was, ‘Which field do I want to study in?’ This question was quickly answered when I took three modules related to energy sources. I immediately learnt about how crucial renewable sources play in transitioning our world to net zero. The idea of being able to contribute directly to society and have the opportunity to work with some fantastic researchers is definitely a ‘pinch me’ feeling!
Can you tell us more about your research?
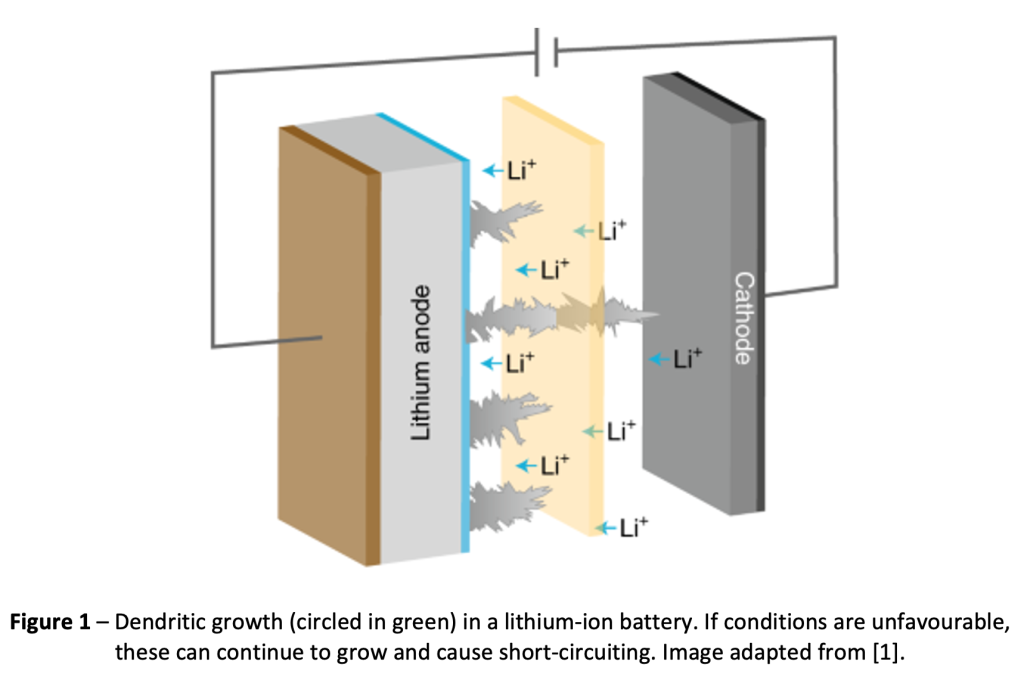
What does a typical day involve?
One of the best parts of a PhD is how varied the days are. It’s essentially a four-year project, which means there are many tasks to get on with and these tasks differ depending on the stage of your PhD and personal deadlines.
Recently, I’ve been spending my mornings in the lab synthesising electrode materials (which involves a lot of stirring) or coin cell batteries. Testing the battery performance immediately after assembling them is always slightly stressful as you can expect at least one to fail! My afternoons are generally spent on my desk, analysing data, making presentation slides or reviewing current literature. My days can also consist of teaching undergraduate students (as a Graduate Teaching Assistant) or supervising master’s students.
Can you tell us more about your research group?
My project ties in electrochemistry with complex materials characterisation techniques and because of this, I have several research groups spanning different research themes. I work primarily under Professor Mary Ryan(Fig. 2a), whose large interdisciplinary group covers nanoscale science and interfaces, including energy materials, bio-sensors and corrosion science.
I also work with Professor Baptiste Gault (Fig. 2b), who spends his time between Imperial and Max-Planck-Institut für Eisenforschung in Düsseldorf and whose research group specialises in APT and correlative TEM for various applications.
My other research groups include the Conroy group, led by Dr Shelly Conroy (Fig. 2c), and the Interfacial Electrochemistry group, led by Professor Ifan Stephens (Fig. 2d). The research of the former focuses on APT and TEM (particularly 4D-STEM strain analysis) and is part of the cryo-EPS facility at Imperial, while Stephens’ group focuses on the large-scale electrochemical conversion of renewable energy to fuels, namely via LIBs, catalysis and fuel cells.

What do you enjoy outside of your PhD?
 I like to unwind from my PhD by trying out new recipes, whether cooking or baking. As science experiments tend to require careful measurements, cooking is generally more flexible and gives me the chance to be slightly more creative. I also enjoy practising creativity through art, especially hyper-realistic drawings and paintings.
I like to unwind from my PhD by trying out new recipes, whether cooking or baking. As science experiments tend to require careful measurements, cooking is generally more flexible and gives me the chance to be slightly more creative. I also enjoy practising creativity through art, especially hyper-realistic drawings and paintings.
I still enjoy the science realm outside of my PhD, often engaging in outreach events, including school presentations and Student Ambassador days for upcoming engineering students. We also have several group lunches/ dinners per year as part of a research group, including the most recent Christmas lunch with the Conroy group (Fig. 3)!
References:
[1] Babu G, Ajayan PM. Good riddance, dendrites. Nature Energy.