
Name: Parul Bishnoi
Position: PhD Student in the Department of Materials
Research Group: Dr Stella Pedrazzini
Research Focus: Understanding the basic mechanisms of Under Deposit Corrosion (UDC), a major problem for industries using steam generators.
What inspired you to study for a PhD? During my school years, I always had a passion for STEM subjects. I gradually became more involved in Materials Science during my bachelor’s studies. I found it fascinating how interdisciplinary it is and how it combines knowledge from different areas such as chemistry, physics, and engineering. Materials Science also resonated with me because it provides progress to various societal challenges.
During my master’s studies, I had the opportunity to do my master’s project as part of an Erasmus program in Dr. Stella Pedrazzini’s research group. I quickly felt at home both in London and within the research group.
Can you tell us more about your research?
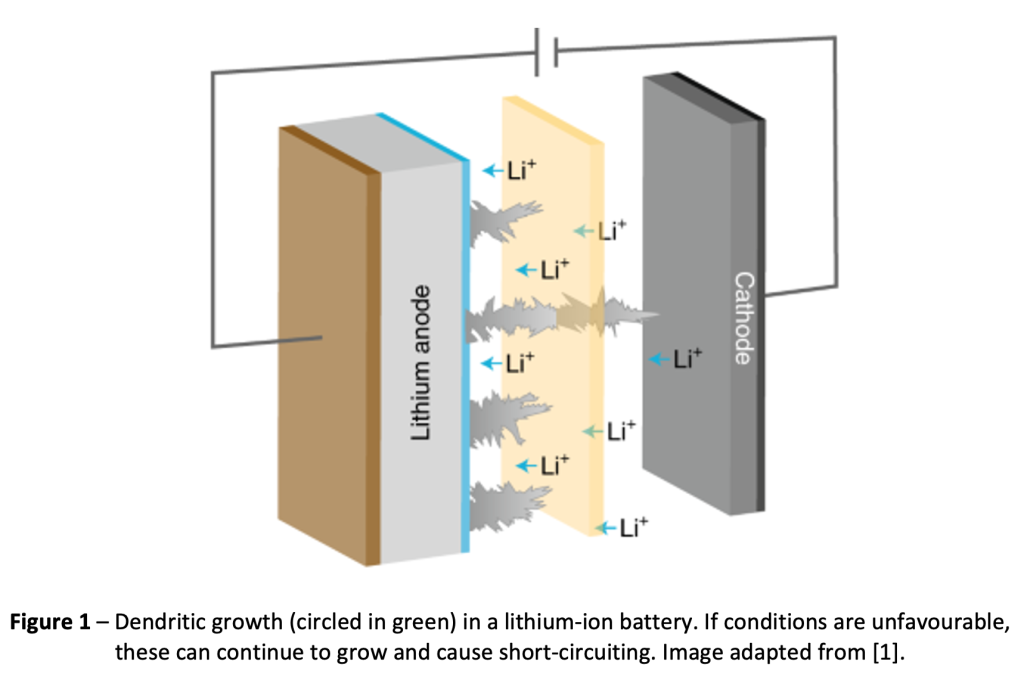
My research investigates the fundamental mechanisms behind Under Deposit Corrosion (UDC). UDC poses significant challenges for industries relying on steam generators. These tubes are susceptible to UDC which occurs beneath deposits, leading to localized and unpredictable damage. Some key characteristics of this corrosion include the presence of porous deposit layers, high concentrations of chloride within these deposits, and the formation of complex laminated corrosion product layers.
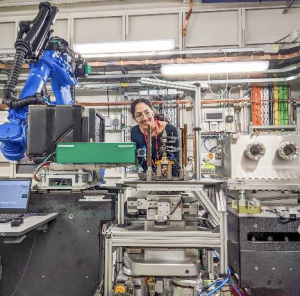
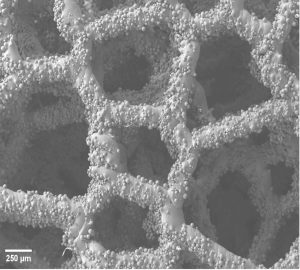
To mimic real-world conditions in the lab, I expose low-alloy steel samples to a nickel chloride solution in pressurized containers, adjusting various parameters. This method aims to recreate the layered corrosion seen in steam generators. Understanding this type of corrosion is tough due to its unpredictable nature and the difficulties in experimenting, but it’s crucial for keeping steam generator components in good shape. Corrosion leads to expensive replacements and higher energy use in steel production, affecting finances and the environment. By studying this corrosion, we aim to improve operating conditions and reduce maintenance time.
What does a typical day involve?
Some days, I spend most of my time in the lab, conducting experiments and using advanced characterization techniques such as XCT (a way to see inside materials using X-rays) and SEM (a microscope that allows us to see tiny details on surfaces) coupled with EDX (a tool to identify elements in a sample) to analyse corrosion products. On other days, I am primarily at my computer, analysing data, answering emails, attending meetings, updating my industrial collaborator on the latest developments, reading papers to stay up-to-date, or working on writing publications or my thesis. I also work as a Graduate Teaching Assistant and am an active Departmental Student Ambassador.
Can you tell us more about your research group?
 I’m part of two research groups. My main supervisor is Dr Stella Pedrazzini and my secondary supervisor is Professor Mary Ryan. My project is supervised by two successful women and I’m fortunate to work in a diverse and inclusive environment. Recently, some of our research group members, including myself, attended the TMS Conference in Florida.
I’m part of two research groups. My main supervisor is Dr Stella Pedrazzini and my secondary supervisor is Professor Mary Ryan. My project is supervised by two successful women and I’m fortunate to work in a diverse and inclusive environment. Recently, some of our research group members, including myself, attended the TMS Conference in Florida.
What do you enjoy outside of your PhD?
Coming from Austria and having grown up in the Alpine foothills, I am passionate about spending time in the mountains. Whether hiking, skiing or mountaineering, being surrounded by nature helps me recharge. Living in London, I enjoy exploring the vibrant city. From visiting museums and galleries to attending theatre performances, there’s always something new and exciting to discover.



 I like to unwind from my PhD by trying out new recipes, whether cooking or baking. As science experiments tend to require careful measurements, cooking is generally more flexible and gives me the chance to be slightly more creative. I also enjoy practising creativity through art, especially hyper-realistic drawings and paintings.
I like to unwind from my PhD by trying out new recipes, whether cooking or baking. As science experiments tend to require careful measurements, cooking is generally more flexible and gives me the chance to be slightly more creative. I also enjoy practising creativity through art, especially hyper-realistic drawings and paintings.





 Dr Jerry Sha is a Research Associate in the group of Professor Stephen Skinner. He joined the Department as an undergraduate student in September 2013.
Dr Jerry Sha is a Research Associate in the group of Professor Stephen Skinner. He joined the Department as an undergraduate student in September 2013. Dr Anna Winiwater is a Research Associate in the group of Professor Ifan Stephens. She joined the Department of Materials this year.
Dr Anna Winiwater is a Research Associate in the group of Professor Ifan Stephens. She joined the Department of Materials this year. Dr Pankaj Sharma is a Research Associate in the group of Professor Fang Xie. He joined the Department of Materials this year.
Dr Pankaj Sharma is a Research Associate in the group of Professor Fang Xie. He joined the Department of Materials this year. Dr Megan Owen is a Research Associate in the group of Professor Sir Robin Grimes. She joined the Department of Materials in 2022.
Dr Megan Owen is a Research Associate in the group of Professor Sir Robin Grimes. She joined the Department of Materials in 2022. Dr Apostolos Panagiotopoulos is a Research Assistant in the group of Professor John Kilner. He joined the Department of Materials in 2018 as a Research Postgraduate.
Dr Apostolos Panagiotopoulos is a Research Assistant in the group of Professor John Kilner. He joined the Department of Materials in 2018 as a Research Postgraduate.
 My research group (the metals electrochemistry group, led by Dr Stella Pedrazzini) is undoubtedly one of the reasons I enjoy doing my PhD so much. We meet once a week to update each other on what we have been up to, whether it is a failed experiment, writing research proposals, or going on holiday!
My research group (the metals electrochemistry group, led by Dr Stella Pedrazzini) is undoubtedly one of the reasons I enjoy doing my PhD so much. We meet once a week to update each other on what we have been up to, whether it is a failed experiment, writing research proposals, or going on holiday! Mike: Workshops are important to produce of a wide range of goods. When you think about it, everything around you is likely created in a type of workshop, from transport to household items and clothing – it just depends on the size, scale, and capability of the facility. In the Department of Materials, we focus on making components that can support research or teaching – and we are always up for a new challenge!
Mike: Workshops are important to produce of a wide range of goods. When you think about it, everything around you is likely created in a type of workshop, from transport to household items and clothing – it just depends on the size, scale, and capability of the facility. In the Department of Materials, we focus on making components that can support research or teaching – and we are always up for a new challenge!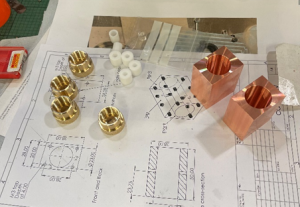 Most parts machined in the workshop are made from metal or plastic. In recent years, we have witnessed a growing demand for the use of new materials such as Peek. Peek is a fairly new material. It has become an increasingly popular material, replacing PTFE for sample holders, as it doesn’t contaminate the material which is being tested, is a more stable material from a machining perspective and is able to operate at higher temperatures.
Most parts machined in the workshop are made from metal or plastic. In recent years, we have witnessed a growing demand for the use of new materials such as Peek. Peek is a fairly new material. It has become an increasingly popular material, replacing PTFE for sample holders, as it doesn’t contaminate the material which is being tested, is a more stable material from a machining perspective and is able to operate at higher temperatures. What do the team most enjoy about their roles in the workshop?
What do the team most enjoy about their roles in the workshop?