In this post, Dr Anusha Seneviratne breaks down the conundrum of cell death and how this process protects our bodies from blood clots.
Your cells die every day. Don’t worry, your body is protecting itself. In a process known as apoptosis or programmed cell death, cells that are no longer needed commit suicide. Some cells are only required for a short time, they may be infected by a virus or develop harmful cancerous mutations. Cell death is also an essential part of development from an embryo. For example, mouse paws begin as spade-like structures and only form the individual digits as the cells in between die. During apoptosis the cells fragment into smaller apoptotic bodies, and their cell surface is flipped open to display lipid molecules called phosphatidylserines, which act as an ‘eat me’ signal to recruit cells called macrophages to engulf them, before their contents spill out and damage the surrounding tissue. This is a process known as efferocytosis.
However cell death is not always so orderly. Some cells suffer premature death known as necrosis, where they burst open for various reasons such as infection, physical trauma or extreme temperatures. As the cell’s contents are released into the open, an inflammatory response is triggered. Studies are ongoing to establish the many mechanisms by which this process occurs. For example, it is known that proteins on the surface of immune cells, called pattern recognition receptors or PRRs, detect the material released by dying cells, and trigger the expression of genes leading to the release of inflammatory substances. When an infection occurs, the immune response is usually short-lived as it rapidly kills the infection. However, when cell necrosis and subsequent inflammation occurs over a longer period, the substances released by macrophages – that have migrated in to engulf the dying cells – can damage the surrounding tissue, resulting in a build-up of dead cells.
It is this damaging chain of events that often occur in atherosclerosis; the build-up of fatty plaques which can block arteries or trigger blood clots leading to heart attacks, strokes or tissue death, known as ischaemia. As fatty lipid molecules, primarily LDL known as ‘bad’ cholesterol, build up in arteries they act like damage signals. Macrophages recognise these damage signals as if it is the phosphatidylserine ‘eat me’ signals, and engulf the lipids to become what is known as a foam cell; a cell full of lipid. A healthy foam cell macrophage can repackage the fatty lipid molecule LDL into larger HDL cholesterol, which is released back into the bloodstream to be excreted by the liver. The healthy foam cell can also leave the atherosclerotic plaque to be disposed of via lymphatic vessels, thus shrinking the plaque.
However, foam cells can become overwhelmed when engulfing excess cholesterol, increasing the release of harmful inflammatory signals, and dying from stress. This typically occurs in individuals who have higher levels of LDL cholesterol in their blood, due to consumption of a diet high in saturated fat and cholesterol, smoking, a lack of exercise and/or diabetes. But all is not lost here. If other macrophages clear the dying foam cells, less harm will be done. The problem is the increased inflammation renders efferocytosis defective, resulting in a process called secondary necrosis. Here apoptotic bodies swell and burst open, as they haven’t been cleared in time. As a result, a large amount of cell debris builds up inside the atherosclerotic plaque, creating what is referred to as a necrotic core. The core is pro-thrombotic when it is exposed to clotting factors in the bloodstream. Thus its exposure increases the likelihood of a blood clot forming in an artery, leading to a heart attack, stroke or tissue death, depending on which artery becomes blocked in the body.
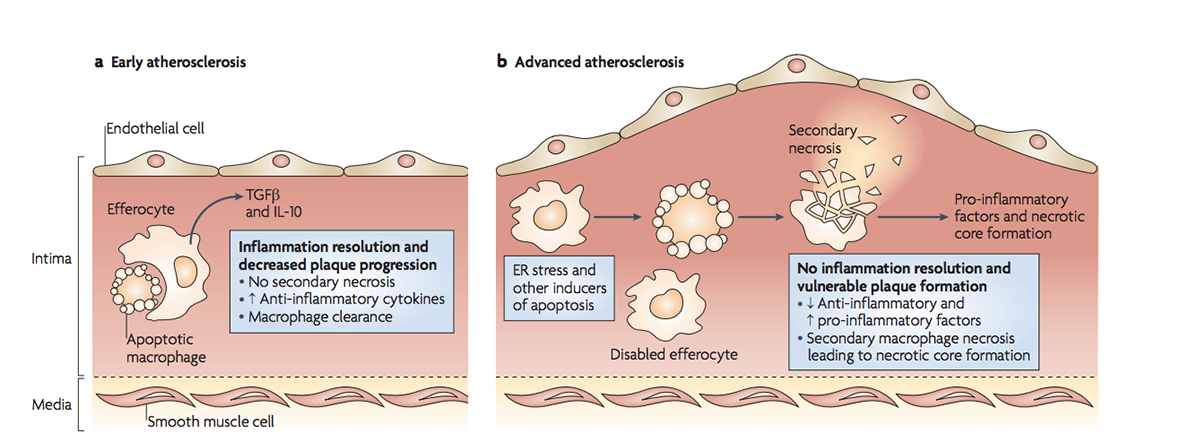
In early atherosclerosis, dying cells are easily cleared. As stress and inflammation increase in advanced plaques, efferocytosis is defective thus secondary necrosis causes necrotic core formation. (Figure 3 from Tabas 2010, Nature Reviews Immunology).
How efferocytosis becomes defective is not entirely understood. During my PhD studies, I was investigating whether inflammation caused by a gene called IRF5 (Interferon Regulatory Factor 5) worsens atherosclerosis as the gene is known to generate inflammatory macrophages. My studies not only confirmed this but led my research group down a fascinating path into its effect on efferocytosis, which was recently published in the high impact journal Circulation.
We examined arteries from tissues where the gene IRF5 had been deleted versus those with normal levels. We already know that deleting IRF5 reduces arthritis and insulin resistance, but our studies showed that even though body weight increased when IRF5 was deleted, there was much less atherosclerosis in the arteries with smaller necrotic cores, and a reduction in genes associated with inflammation.
In fully developed human plaques, IRF5 was present in cells near the necrotic core suggesting it is important for necrotic core formation. This was confirmed when we found cells lacking IRF5 were more resistant to programmed cell death and more efficient at tidying up through efferocytosis. The ability of these cells to perform efferocytosis was dependent on two proteins – Itgb3 and Mfge8, which were both reduced by IRF5. These two proteins form part of a bridge between the macrophage and phosphatidylserine on apoptotic cells.
Recent media attention highlighted the results of a clinical trial for Canakinumab (an antibody that blocks the action of the inflammatory substance Interleukin-1b, and is currently used to treat a range of inflammatory diseases), which showed a reduction in deaths from heart attacks and strokes. While this is a promising result, it is not clear what other unintended effects could occur in the rest of the body. For example, the rate of infection in those taking Canakinumab was double the infection rate in the placebo control group, as Interleukin-1b is important for the normal function of the immune system throughout the body. Our findings could lead to the development of therapies to reduce atherosclerotic plaque formation and limit the chances of thrombosis, without targeting such an important component of immune function. However, IRF5 is important to fight viruses, thus further studies are needed to establish to what extent blocking IRF5 can enhance efferocytosis and reduce heart disease risk, without compromising the battle against viral infection.
Dr Anusha Seneviratne is a Research Associate in the Macrophage Differentiation Group ,Vascular Sciences Section, National Heart and Lung Institute (NHLI).