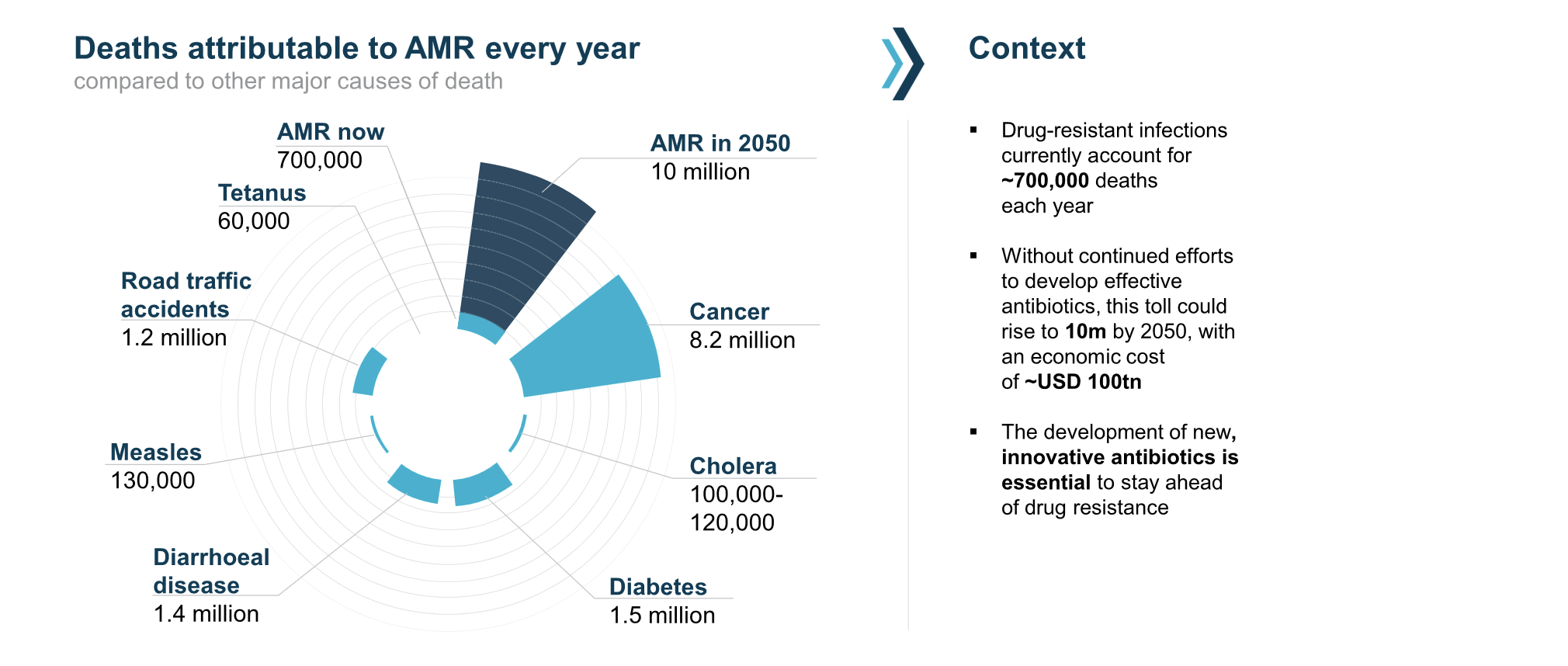
Dr Cheryl Battersby, Clinical Senior Lecturer in the School of Public Health and theme lead for Neonatal Medicine at Imperial’s Centre for Paediatrics and Child Health (PaeCH), shares how neonatal research is helping to shape lifelong health outcomes for the most vulnerable babies. From harnessing the power of national data to leading pioneering research programmes, Cheryl and her colleagues are working to ensure that every baby – no matter how early or unwell they are born – has the best possible start in life.
We believe that every child deserves the best start in life, and we know from decades of research and clinical care that the health of a baby in the first days and weeks after birth can shape their entire future.
In the UK alone, around 90,000 babies each year are admitted to neonatal units. Some are born too soon. Others arrive on time but need urgent medical care. These fragile early moments matter. They are the beginning of a lifelong journey.
At Imperial College London, our Neonatal Medicine Research Group is one of the largest academic neonatal centres in the UK. We’re a dynamic team of neonatologists (doctors who specialise in the care of newborn infants), neonatal nurses, statisticians, data scientists, and public involvement experts – working together to transform care for the smallest, sickest newborns.