
In this post, Jennifer Shelton provides an insight into her PhD project which involves over 500 citizen scientists from across the world, in the hope of better understanding a species of fungus that is linked to disease.
It started by a poolside in Gran Canaria.
I was reading my book but thinking about the sticky films we use in the lab to cover plates of DNA that a former postdoc in my group had used to collect Penicillium spores for his study on the population genetics of ‘Alexander Fleming’s lucky fungus’. I’d already decided as part of my PhD to conduct a country-wide survey to determine background levels of Aspergillus fumigatus – a species of fungus – spores in the UK. I had put aside several weeks for driving around the country to collect air and soil samples, yet thoughts of a citizen science project kept buzzing. What if I asked individuals across the UK to collect samples of their local air on a single day, say summer solstice, and post them back to me?
Citizen science projects are increasing in popularity and rely on members of the public to voluntarily collect samples, process data or record observations as part of a research project. Some well-known examples include SETI@Home, which uses internet-connected computers to analyse telescope data in the search for extra-terrestrial life; Foldit, an online video game about protein folding and Swab & Send, a widespread swabbing exercise to identify novel antibiotics in the environment.
An idea was born
I went straight to work and mocked up a poster design in my hotel room. Two weeks later the project went live on Twitter and Facebook when I tentatively shared the poster and sign-up link, crossing my fingers for 100 participants. The response was phenomenal! Within 72 hours of launching I had 166 sign-ups from 40 counties across the UK. Within five weeks I disabled the sign-up link as I had reached 485 UK participants and 66 global participants, and my Tweet had been viewed by over 22,000 people! I then set about packing and posting air sampling kits to all 551 participants across the country and world.
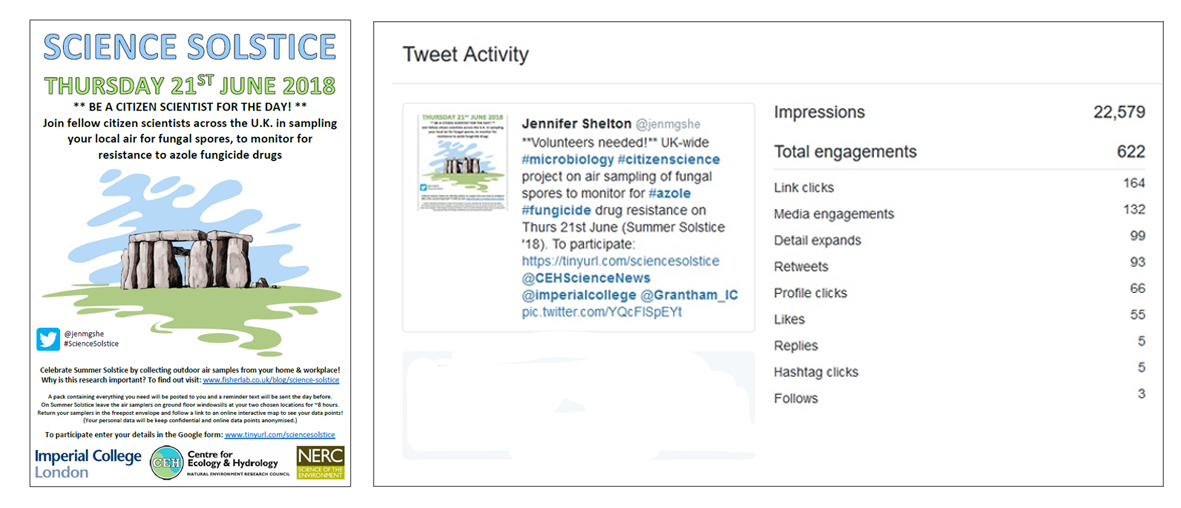
Why study spores?
The reason for surveying background levels of A. fumigatus spores is because we all breathe in hundreds of these spores every day. It is a ubiquitous fungus found indoors and outdoors, in air and soil, and on most continents and performs an important role as a decomposer. These spores pose no risk to the majority of individuals as they are cleared by our innate immune system, but some individuals suffer debilitating allergic responses caused by hypersensitisation to the inhaled spores. This is known as allergic bronchopulmonary aspergillosis or ‘fungal asthma’.
A. fumigatus also causes illness if it establishes and grows within different locations in the lung, such as the cavities or airways. Invasive Aspergillosis (IA) is a severe lung infection that affects immunocompromised individuals, such as those with HIV/AIDS and those receiving chemotherapy or organ transplant therapy. IA requires long-term treatment with a number of azole class antifungal drugs and has a 40-90% fatality rate depending on patient history. Increasingly, IA patients are infected with A. fumigatus that is resistant to one or more of these drugs, which results in alternative treatment regimens being sought and, unfortunately, higher fatality rates. A. fumigatus can develop resistance in the lung due to prolonged treatment with these drugs but patients also present with resistant infections despite no prior treatment with medical azoles. As there is no evidence of patient-to-patient transmission of IA, this suggests acquisition of resistant A. fumigatus from the environment.
Agricultural fungicide sprays often contain azole compounds so one theory is that A. fumigatus in the environment is being exposed to these azoles in farmers’ fields and that a percentage of the spores we’re inhaling have already evolved resistance. To test this, I want to collect air samples at head height from as many locations across the UK as possible and determine whether the proportion of resistant spores correlates with proximity to crops sprayed with azole fungicides.
How did participants take part?
I asked everyone to secure the air samplers sticky-side up on ground floor windowsills at two locations, i.e. home and workplace, for a period of 6-10 hours on Thursday 21 June summer solstice 2018. They then re-covered the samplers and posted them back to me in a freepost return envelope, along with a questionnaire containing details of the two locations. I was advised at a citizen science symposium that the generally accepted response rate is 10-15% so I was optimistic and prepared myself in the lab to receive air samplers back from ~100 individuals.
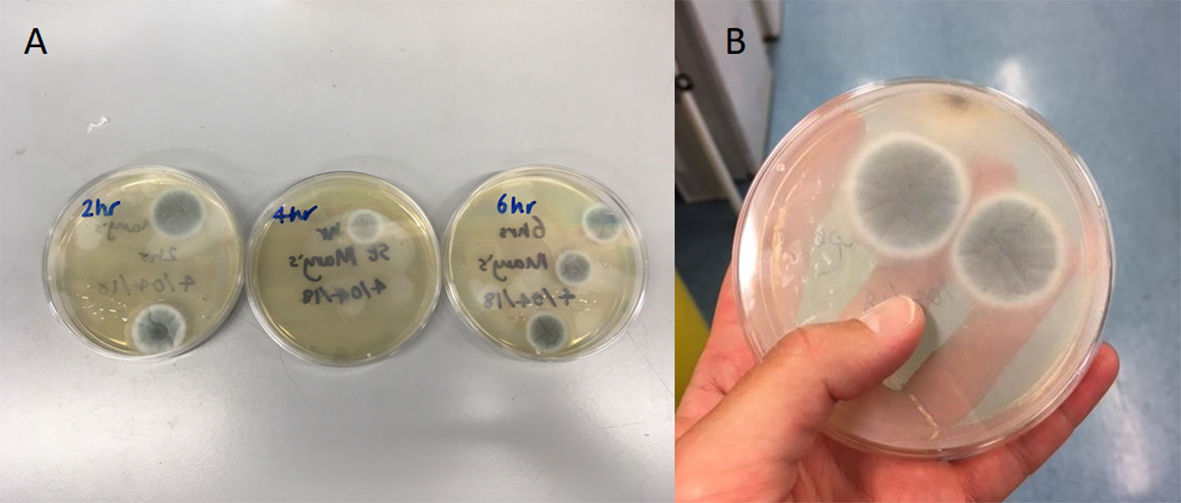
How wrong I was! In the fortnight following summer solstice I received envelopes back from 425 individuals (77% response rate!), which equated to 864 air samplers. I incubated these air samplers on agar plates and they grew an incredible 1,431 A. fumigatus colonies, which are now preserved and refrigerated awaiting resistance testing.
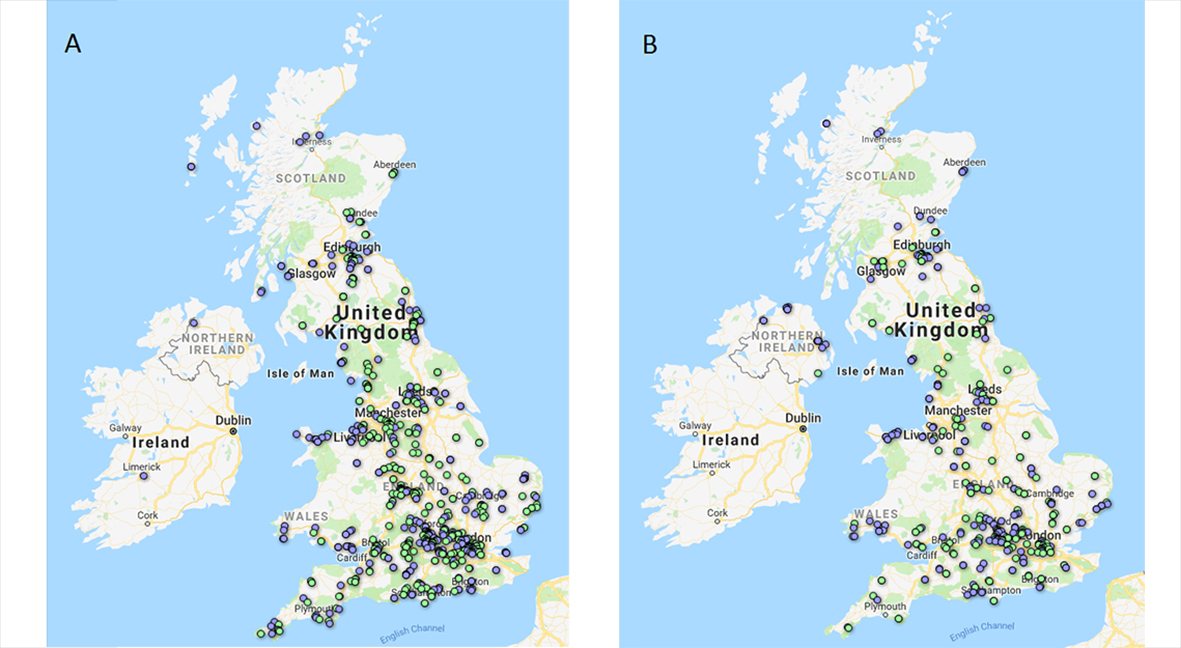
#ScienceSolstice success
Due to the enormous success both in terms of response rate, colonies grown and in the enthusiasm and positive feedback from participants, I asked if everyone was willing to repeat the sampling on a further three dates. These dates are Monday 24 September (autumn equinox 2018 + 1), Friday 21 December (winter solstice 2018) and Wednesday 20 March (spring equinox 2019), with the intention of seeing if season has an impact on number of spores collected or proportion of spores that are resistant. #AutumnAirquinox again garnered huge support, with 306 sampling packs sent out and an impressive 254 returned (83% response rate). This equated to 490 air samplers, which grew a total of 600 A. fumigatus colonies.
Each colony is to be transferred to an agar plate containing tebuconazole – the third most used azole in agriculture in the UK – and a control plate without azole. If the colony grows on the control plate and not on the tebuconazole plate then it is not resistant, but if it grows on both this indicates the spores have a mutation that confers resistance to azole drugs. It is unknown whether there will be any resistant spores circulating in the air, but if there are I am interested to know if there is spatial clustering of resistance in agricultural areas with high azole spray rates. This may identify ‘hot spots’ of resistance in the UK that individuals at highest risk of contracting IA could be advised to avoid.
This project is an example of the powerful role citizen science can play in national, and even international, monitoring for antimicrobial resistance (AMR) in the environment. There is no way I alone, or even my research group together, could have collected this many samples from all over the world on a single day to achieve the coverage of this project. I am incredibly grateful to all the individuals who have participated so far; for collecting the samples, for their feedback and suggestions for improvement, for their wonderful messages of support, and for their genuine interest in my research. Based on my experience, I would urge other researchers to consider whether citizen science can be incorporated into their research to enhance public participation in science and to achieve widespread environmental sampling.
If you would like to be involved in future sampling rounds please contact me, or if you are interested in project updates please keep an eye on the Fisher Lab Blog.
Jennifer Shelton is a PhD student supervised by Professor Matthew Fisher at Imperial’s Department of Infectious Disease Epidemiology, in the School of Public Health, and Dr Andrew Singer at the Centre for Ecology and Hydrology (CEH) in Wallingford, Oxfordshire. She is part of the Science and Solutions for a Changing Planet (SSCP) DTP run by the Grantham Institute and funded by NERC.
Amazing research!!!
as a sufferer of ALPHA1 and immuno compromised I am affected by fungus, moulds, chemicals as we all are
and am particularly interested in lungs and the environment an issue for us all
I would love to see this study done in Australia.