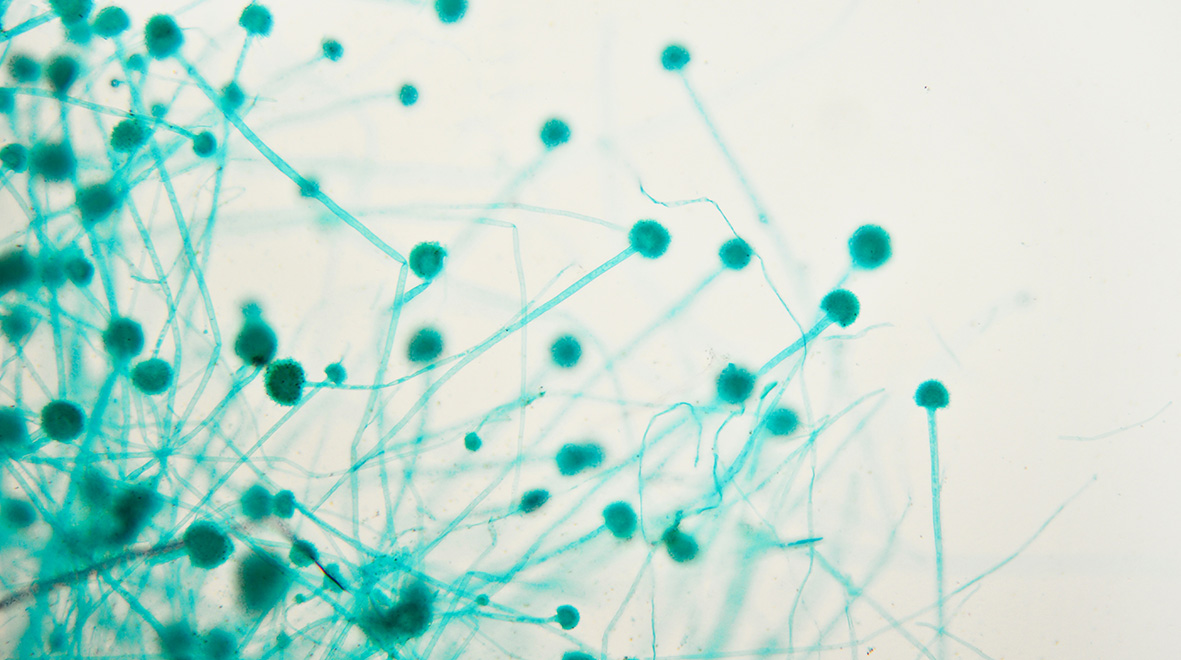
Jenny Shelton highlights the potential for invasive and chronic fungal lung infections with Aspergillus fumigatus in COVID-19 patients and the dangers posed by growing antifungal resistance.
Virtually unknown just a few months ago, the COVID-19 pandemic has affected millions worldwide. The pathogen responsible, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), infects alveolar cells in the lungs. Parallels are already emerging between severe COVID-19 infection and severe influenza. Influenza, or ‘the flu’, is also caused by a virus that infects cells along the respiratory tract and is associated with similar symptoms to COVID-19 but has a lower death rate (<0.1%). Studies have found that up to 65% of individuals hospitalised with severe influenza infection are co-infected with bacteria. A recent review found 9 studies, undertaken in China and USA, that reported bacterial coinfection in a combined 62 of 806 (8%) individuals admitted to hospital with COVID-19 infection and the majority of patients (72%) received antimicrobial drugs.
Another secondary infection associated with severe influenza is invasive pulmonary aspergillosis (IPA), which develops when spores from the fungus Aspergillus fumigatus grow in the lung and pass into the bloodstream to cause sepsis. IPA is diagnosed in up to 19% of individuals hospitalised with influenza, with significantly higher mortality in the patients with IPA.
What this means for COVID-19 patients
Evidence is starting to emerge that IPA could present a complication in patients with COVID-19. Although the numbers are small (n = 27 patients), an online preprint suggests that 33% of COVID-19 patients in France that were admitted to intensive care units (ICU) had evidence of aspergillus coinfection. Another study, undertaken in Germany, found IPA in five of 19 COVID-19 patients with acute respiratory distress syndrome (ARDS). There is also evidence of fungal coinfections from COVID-19 patients in China and administration of antifungals has been reported in 15% of patients. These early data suggest that individuals with severe COVID-19 infection need to be closely monitored for both fungal as well as bacterial coinfections and treated accordingly to prevent associated morbidity and mortality.
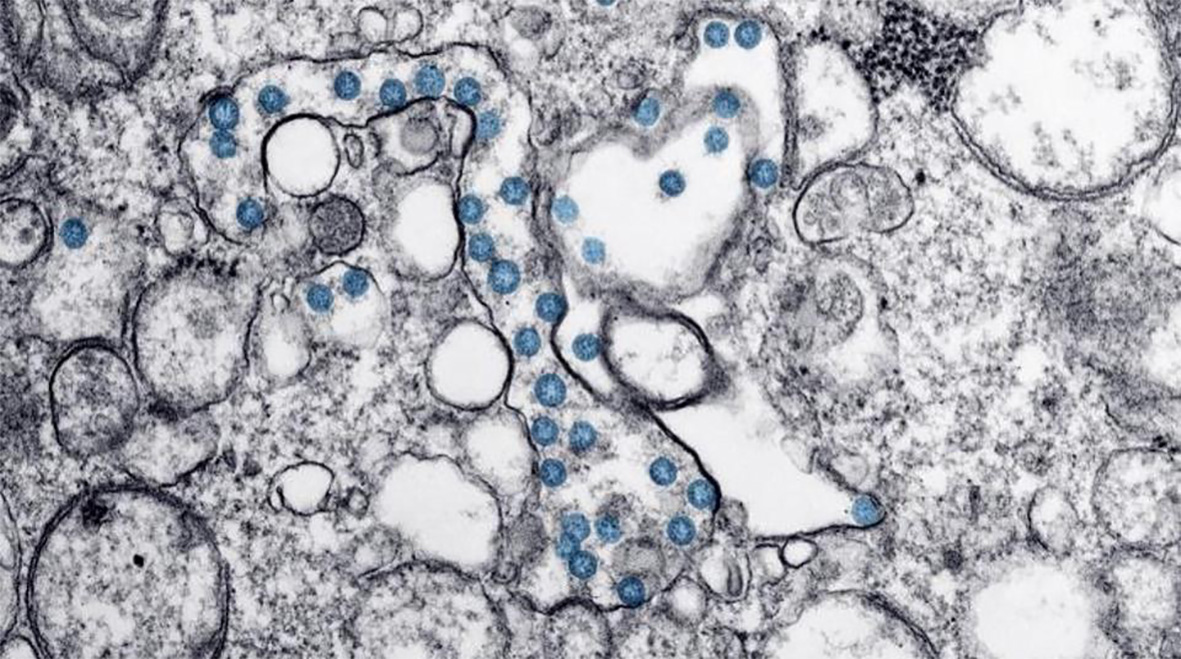
Several articles have already highlighted the danger of antibiotic resistance resulting from mass administration of antibiotics to COVID-19 patients, and the National Institute for Health and Care Excellence (NICE) have issued stewardship guidelines to avoid inappropriate or excessive use of antibiotics during this pandemic. If antibiotics are not used carefully to treat bacterial infections of the respiratory tract we face losing more drugs to widespread resistance, which will have knock-on effects on treating other diseases such as bacterial sepsis, sexually-transmitted infections (STIs) and urinary tract infections (UTIs).
The same is true for antifungal drugs
Antifungal drugs containing azoles are used to treat IPA infections and clinical azole resistance has been reported in the UK, Europe, Asia and the Middle East. In the UK alone, the percentage of patients with azole-resistant IPA has increased from 14% in 2008 to 20% in 2009. Resistance can develop after long-term treatment with azole drugs and resistant infections are associated with treatment failure and increased mortality.
IPA is an acute and invasive form of aspergillosis disease that is associated with immunosuppression or active influenza infection, and there are also chronic aspergillosis diseases that stay localised to the lungs. Risk factors for chronic pulmonary aspergillosis (CPA) and aspergillomas (“fungal balls”) are severe asthma, smoking, chronic obstructive pulmonary disease (COPD) and previous influenza or TB infection as these can all cause lung damage and disruption of the lung immune system. As a result, A. fumigatus spores that would normally be cleared by our innate lung defences can penetrate deeper into the lung and grow; causing fever, weight loss, persistent cough, chest pain and difficulty breathing. As severe COVID-19 infections cause serious lung damage, we may well see an increase in aspergillosis infections in recovered patients in the longer term.
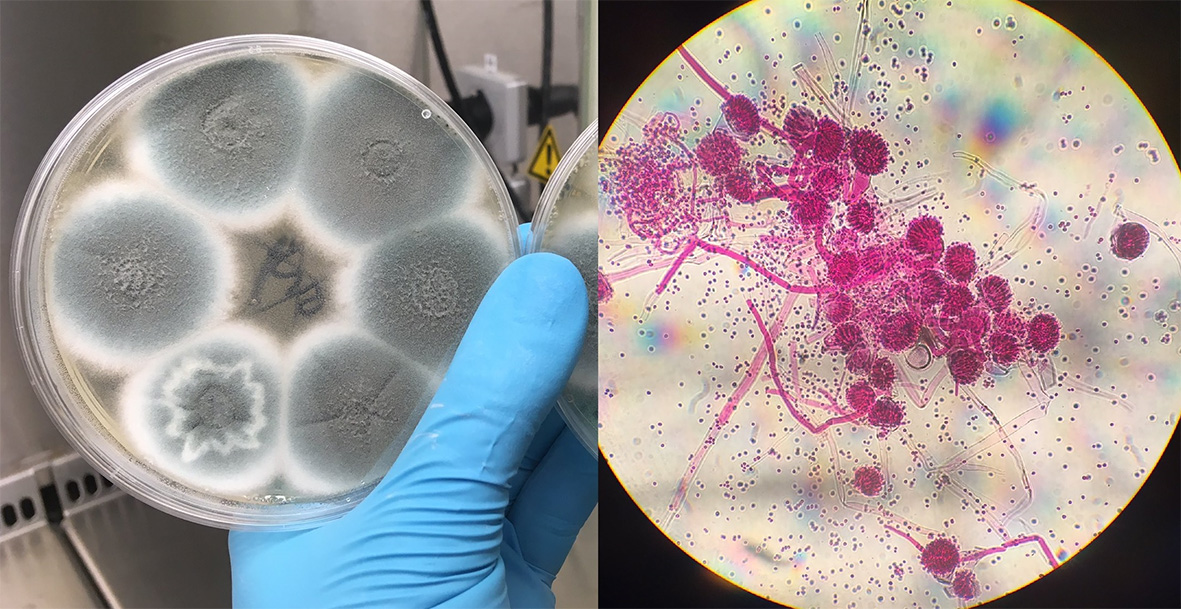
Airborne spores
Aspergillosis infections result from breathing in A. fumigatus spores in our environment. These spores originate in the soil and are carried on air currents; they are found everywhere and it is estimated we inhale several hundred every day. Not all individuals who develop drug-resistant aspergillosis have been treated with azole drugs before, which suggests the spores were already resistant when they inhaled them. Azoles are commonly used in agricultural fungicides to protect crops from fungal pathogens and in material preservation, so it is likely that spores acquire resistance from exposure to these azoles in the environment.
On four dates between June 2018 and March 2019, citizen scientists living in the UK collected air samples from their homes and workplaces to capture A. fumigatus spores. Of the 2,366 spores captured, 4.5% were resistant to tebuconazole and had cross-resistance to medical azoles itraconazole, voriconazole and isavuconazole (unpublished). Voriconazole is recommended as the front-line treatment for IPA and itraconazole for chronic aspergillosis, but with at least 1 in 20 infections likely to be resistant to these drugs going forward medical professionals need to look out for treatment failure and be ready to switch to salvage therapy with high-dose posaconazole as soon as possible.
Clinical response
As the road to eliminating COVID-19 is long and unclear, there will be a growing population of individuals that are susceptible to aspergillosis disease making increased testing, prompt diagnosis and suitable treatment crucial to improving patient outcomes. Medics need to be aware of the challenge of growing antifungal resistance and have plans in place should patients fail front-line treatment. Stewardship guidelines surrounding antibiotic use need to include antifungals. Importantly the agricultural and industrial use of azoles should be subjected to the same scrutiny as antibiotic use in farming, and novel antifungals that have been developed for use in humans should be ring-fenced for exclusive use in medicine if we are to prevent fungi joining the growing Pantheon of ‘superbugs’ in a post-COVID-19 era.
By Jennifer Shelton, Professor Matthew Fisher and Dr Andrew Singer
Jennifer Shelton (@jenmgshe) is a PhD student with Professor Matthew Fisher in Imperial’s Department of Infectious Disease Epidemiology (DIDE) and Dr Andrew Singer at UK Centre for Ecology and Hydrology (UKCEH). She is part of the Science and Solutions for a Changing Planet (SSCP) DTP run by the Grantham Institute and funded by NERC.
Further resources on aspergillosis:
The Aspergillosis Trust – a patient support group raising awareness of aspergillosis disease
National Aspergillosis Centre – the first national centre for the treatment of aspergillosis
Great blog post helfpul and informative.I am always read your blog . I like it thanks for sharing this information with us .