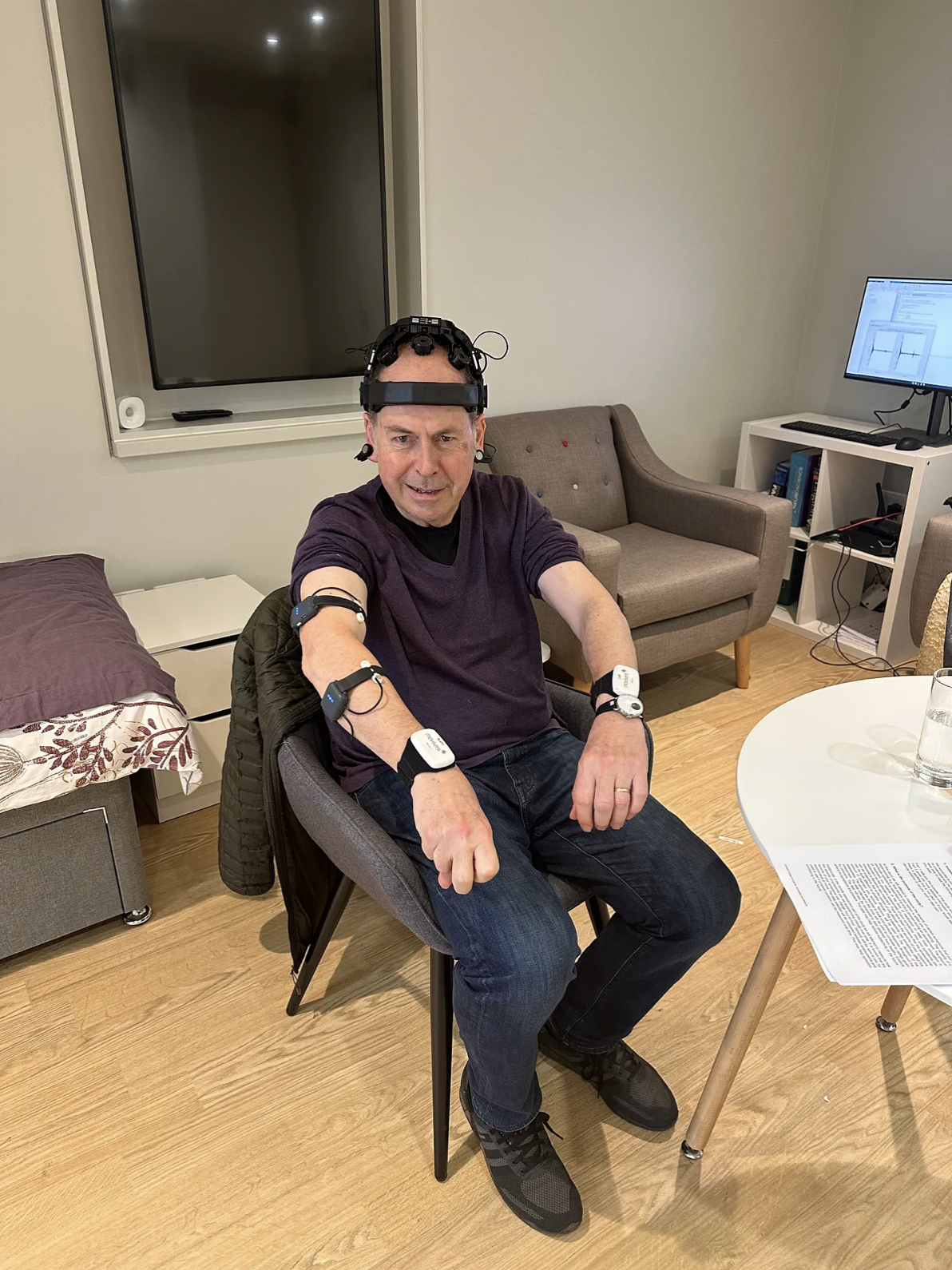
Rory Cellan-Jones is an author and former BBC Technology Correspondent who, in 2019, was diagnosed with Parkinson’s Disease. Rory discusses his visit to the ‘Living Lab’ at the UK DRI Care Research & Technology Centre – a unique mock apartment where scientists can monitor the behaviour of patients in a domestic environment.
My week started with quite a stressful day. For nearly five hours I was under the microscope, my every move watched by scientists. They made me walk up and down, rise from a chair without using my arms, open and close my hand rapidly. I spent half an hour staring at a computer screen trying to work out which shape fitted where on a grid, one of a number of cognition tests. They even made me make two cups of tea and four slices of toast.
It was tiring but it was all in the cause of science – and potentially faster drug trials. This all took place in Imperial College’s Living Lab, a room fitted out like a small flat on the ninth floor of a tower block in West London. The lab is equipped with video cameras and a series of sensors which provide data on its occupants’ activities.
It is part of the UK Dementia Research Institute which is trying to develop technology which would allow people with dementia to be monitored so that they can live at home longer. But there is also a project to find ways of measuring the symptoms of Parkinson’s more accurately which, as I’ve explained here before, is vital if we are to know whether new drugs are working.
I was told to arrive at the lab at 11, the time I take my second daily round of Parkinson’s drugs. Then I was fitted out with half a dozen wearable sensors and a helmet contraption which made me look like the Terminator but which measured my brain waves. By that time the drugs had kicked in and one of the team, a doctor who works with my consultant at Charing Cross Hospital, gave me the standard UPDRS tests which I have at every appointment to assess my Parkinson’s symptoms.

Meanwhile, the cameras and sensors were producing data to compile an automated version of the tests which will then be compared to the score given by the doctor. People without Parkinson’s will also be monitored in the lab, and the aim is to crunch all this data to develop a less subjective analysis of the progression of the disease .
Next, the cameras and sensors were treated to an exercise where I made cups of tea and slices of toast according to a strict protocol described to me by the researchers – for instance, I was not allowed to take a swig of my tea while I made the toast because that wasn’t in the protocol.
My mental strength was also tested in the cognition tests. Counting back in sevens from a hundred, naming the current Prime Minister, and even the last one, went fine – remembering where on a grid the chicken was in relation to the dice proved a lot more challenging.
Then as 3pm approached and my next round of drugs was due, I was asked to make tea and toast again – in theory, with the effects of the 11am pills wearing off, my ratings for effective work in the kitchen should have been quite a deal worse.
Finally, exhausted by my efforts, I was able to remove the headset and sensors and head for home. It had been fun but the next day I called the man running the project Dr Shlomi Haar to get a better idea of what benefits could come to people with Parkinson’s from the research. He said there could be a huge prize – shorter and cheaper clinical trials for new drugs:
“If we can assess more accurately, and I can detect change in disease progression not after two years of study, but after three months of study, then it completely revolutionises clinical trials both in terms of cost and in terms of the patients needed. I would need less participants for each clinical trial because I’d have much more conclusive results.”
The importance of this was hammered home to me by a news story which broke the day I was at the lab. Parkinson’s UK revealed that a trial of an experimental drug designed to deal with one of the worst side-effects of levdopa had shown promising results. NLK-111 had proved safe and effective in combating dyskinesia, the involuntary jerking movements that many people suffer after taking the main Parkinson’s medication.
This seemed like exciting news – until I read on and found that the drug had only passed a 2a trial – I hadn’t even know that phase 2 trials could be split into two stages. Apparently a 2b trial will take longer and require more patients and then there is probably a long gap to phase 3 and we could be talking 2030 before doctors are prescribing the drug. So let’s hope that Dr Haar and his team at the Living Lab come up with technology that can get such trials moving at warp speed rather than at the funereal pace we see today.
Find out more about the work of Dr Shlomi Haar from Department of Brain Sciences and a UK DRI Emerging Leader at the UK Dementia Research Institute Care Research & Technology Centre.
This blog originally appeared on Rory’s Always On Newsletter.